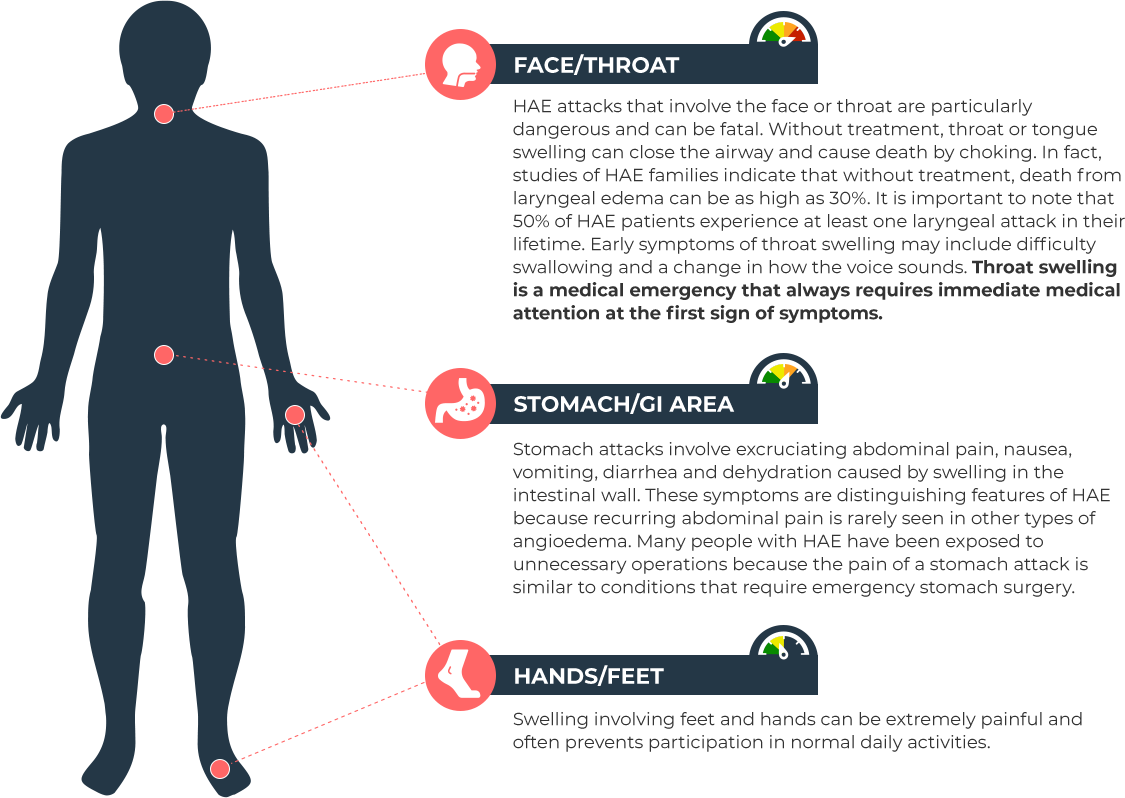
Hereditary Angioedema, or HAE, is a very rare and potentially life-threatening genetic condition that involves recurrent attacks of severe swelling (angioedema) in various parts of the body, including the hands, feet, genitals, stomach, face and/or throat. Swelling in the airway can restrict breathing and be fatal. Episodes may be triggered by physical trauma or emotional stress, however, swelling often occurs without a known trigger.
Symptoms of HAE usually appear early in life, most often by age 13, and may increase in severity after puberty. Because HAE is so rare, it can take as long as a decade to obtain an accurate diagnosis after symptoms are first experienced.
When untreated, an HAE attack often lasts for three days, sometimes even longer, and many people with HAE experience three or more swelling attacks per month. The frequency and severity of attacks vary significantly among individuals, even among affected family members.
The vast majority of people with HAE have a genetic defect that causes a deficiency in the plasma protein called C1-Inhibitor. HAE is also seen in people who have normal levels of C1-Inhibitor, however, genetic defects in other genes cause their angioedema.
People with HAE experience recurrent episodes of swelling in the hands, feet, genitals, stomach, face, and/or throat that can last from two to five days. The frequency and severity of attacks may vary dramatically in people affected by HAE, even those in the same family. About 25% of people with HAE experience a flat, non-itching red rash that often occurs before or during an HAE attack.
The vast majority of people with HAE have a defect in the gene that controls a blood protein called C1-Inhibitor. This defect causes a biochemical imbalance that produces swelling. HAE is also known as C1-Inhibitor Deficiency - Type I and Type II. People diagnosed as having HAE with Normal C1-Inhibitor experience swelling symptoms generally similar as seen in Type I and II. US HAEA is actively funding research designed to better understand the underlying genetic and biochemical causes and find treatments for the very important members of our community who suffer from HAE with Normal C1-Inhibitor.
HAE is hereditary and children have a 50% chance of inheriting HAE if one of their parents has the condition. However, the absence of a family history does not rule out the diagnosis of HAE, as scientific reports indicate that as many as 25% of HAE cases result from a spontaneous mutation of the C1-Inhibitor gene at conception. Children of people with HAE may also inherit the condition.
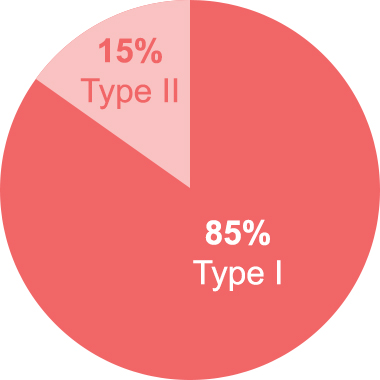
HAE is considered a very rare disease, and occurs in about 1 in 10,000 to 1 in 50,000 people. Type I is the most common, accounting for approximately 85 percent of cases. Type II occurs in approximately 15 percent of cases. There is no data regarding the incidence of HAE with Normal C1-Inhibitor, however, expert scientists believe that the population represents a small subset of people who suffer with HAE.
While some attacks appear to be spontaneous, some of the more common causes of HAE attacks are:
People with HAE have also reported swelling in extremities following:

 HORMONES
HORMONES ACE INHIBITORS
ACE INHIBITORS DENTAL PROCEDURES
DENTAL PROCEDURES MOST cases of angioedema or swelling are NOT HAE or C1-Inhibitor deficiency.
HAE is very rare, and most people – including medical professionals – are unfamiliar with the disease until they encounter someone who has it. Even then, as symptoms come and go, it is common for people with HAE to remain undiagnosed for many years. Frequent and severe abdominal pain can easily be misdiagnosed, sometimes even resulting in unnecessary exploratory surgery.
Proper diagnosis is crucial for successful treatment and management of HAE. Laboratory analysis of blood samples, or genetic samples, are required to establish an HAE diagnosis. There are three specific blood tests used to confirm Hereditary Angioedema Type I or II:
1. C1-Inhibitor quantitative (antigenic)
2. C1-Inhibitor functional
3. C4
Genetic testing for HAE with Normal C1-Inhibitor can determine if there is a defect in one of the other three genes that have been shown to also cause HAE.